The Periodic Table: searching for order
History of the Periodic table:
Research on of the following who contributed to the development in ordering the elements.
Put your findings in a PowerPoint presentation to present to the rest of the class:
| Aristotle | The 4 elements |
| Antoine - Laurent de Lavoisier | The list of elements |
| Jons Jakob Berzelius | Symbols and Atomic weights |
| Johan Wolfgang Dobereiner | Law of Triads |
| Alexandre-Emile Beguyer de Chancoutois | Spiral cylinder / aligning similar elements |
| John Newlands | Law of Ocataves |
| Dmitri Mendeleev | Atomic masses / Periodic law |
| Henry Moseley | Atomic number |
| Glenn Seaborg | Transuranic elements 94 - 102 |
The Periodic table: Mendeleev and beyond
The Periodic Table is the Bible to Chemists. It brings order and a systematic way of looking at the elements.
Prior to the periodic table, it was very difficult to find patterns in the elements.
Although many people contributed to the development of the modern Periodic table, it falls mainly down to a handfull:
Dobereiner – Law of Triads:-
If you look at the properties and relative atomic masses of 3 elements in group 1:-
|
Element |
RAM |
Properties with water |
|
6.9 |
Fizzes gently – hydrogen given off |
|
|
23 |
Fizzes – hydrogen given off |
|
|
39.1 |
Fizzes violently – hydrogen given off |
The RAM of sodium is the mean of lithium and potassium.
The rate of reaction of sodium is also in between the other 2.
This also occurs for other ‘triads’ of elements.
Newlands – Law of Octaves:-
Newlands was the first to notice that if you arrange the elements in order of RAM, every 8th element exhibited similar physical and chemical properties.
This only worked for the fist 16 elements and other elements were missing.
| Na | Mg | Al | Si | P | S | Cl | Ar | K |
Mendeleev’s Periodic Table
Mendeleev was the first to put the 60 known elements in the arrangement that they are in today.
Mendeleev arranged the elements in increasing mass order but left ‘gaps’ for elements that had ‘not been discovered yet’.
Although there were only 60 elements at the time the, gaps he left made it possible to predict the properties of elements that had not yet been discovered. (Like an incomplete jigsaw)
Mendeleev’s prediction about Gallium (not yet discovered) was incredibly close:-
|
Mendeleev's Prediction |
Observed Properties |
|
Atomic mass = 72 |
Atomic mass = 72.6 |
|
Density = 5.5gcm-1 |
Density = 5.35gcm-1 |
|
Silvery grey colour |
|
|
Will combine with 2 atoms of oxygen to form a white powder (the oxide) with a high melting point. |
Combines with 2 atoms of oxygen to form a white powder (the oxide) with a melting point above 1000oC |
|
Oxide density = 4.7gcm-3 |
Oxide density = 4.2gcm-3 |
|
Will combine with 4 atoms of chlorine to form a chloride with a boiling point less than 100oC. |
Will combine with 4 atoms of chlorine to form a chloride with a boiling point less than 100oC. |
|
Chloride density = 1.9gcm-3 |
Chloride density = 1.8gcm-3 |
These predictions were 17 years before Gallium was discovered and shows the correctness of his ideas.
Henry Moseley
Moseley showed that the real order of the elements in the Periodic Table is based on atomic number and not RAM.
The sequence is close but not exactly the same.
Questions P79 1 - 2 / P81 1 - 2
The elements are arranged in increasing atomic number.
Elements with similar properties appear in a regular pattern in the list
|
|
Groups:
A vertical column is called a group:- Groups are numbered I - VII, and 0. Some groups have names as well.
Groups often show an increase or decrease in similar properties.
Periods:
A horizontal row is called a period:- Periods are numbered from the top downwards (1-7).
Periods often show gradual changes in properties.
These patterns are repeated across each Period.
This is called periodicity
Periodicity:
Is a regular periodic variation of properties of elements with atomic number and the position in the Periodic Table
Trends in properties can occur across periods and down groups:
Across a Period: Metal à Non - metal
Down Group 4: Non - Metal à Metal
Variation in electron structure:
Chemical reactions are due to the outer shell electrons only.
This means that those elements with the same number of electrons in the outer shell will react in similar ways.
The vigour of the similarities will depend on shielding and the number of protons in the nucleus (Li - Cs).
This is what causes Periodicity - the regular repeating patterns:
| Li | Be | B | C | N | O | F | Ne |
| [He]2s1 | [He]2s2 | [He]2s22p1 | [He]2s22p2 | [He]2s22p3 | [He]2s22p4 | [He]2s22p5 | [He]2s22p6 |
| Na | Mg | Al | Si | P | S | Cl | Ar |
| [Ne]3s1 | [Ne]3s2 | [Ne]3s23p1 | [Ne]3s23p2 | [Ne]3s23p3 | [Ne]3s23p4 | [Ne]3s23p5 | [Ne]3s23p6 |
Questions 1-2 P83
Periodicity: Ionisation energies and atomic radii
A) Trends across a period:
1) Periodic Patterns: Atomic radii
As you go across a period, the nuclear charge increases.
And the shielding between nucleus and outer electron shell remains the same.
The increased nuclear charge pulls the electron shell closer to the nuclei.
This means there is a slightly greater attraction between the nucleus and the outer shell electrons.
The increase in nuclear charge is the most important factor.
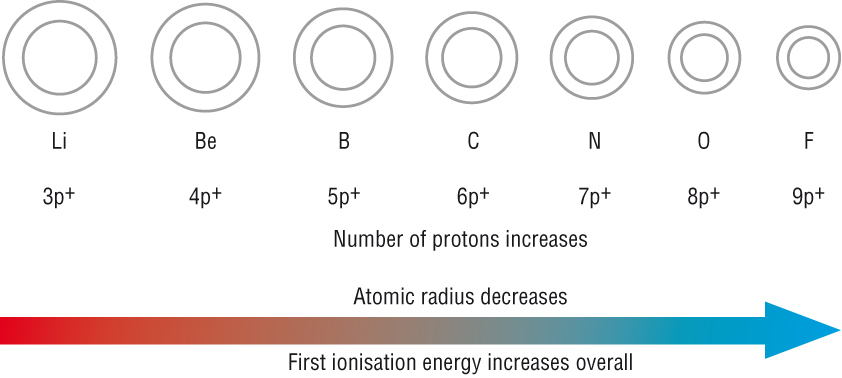
2) Periodic Patterns: First ionisation energies
The explanation for atomic radii explains the trend in first ionisation energies across a period
Graph of 1st ionisation energies for the first 36 elements: SEE GRAPH
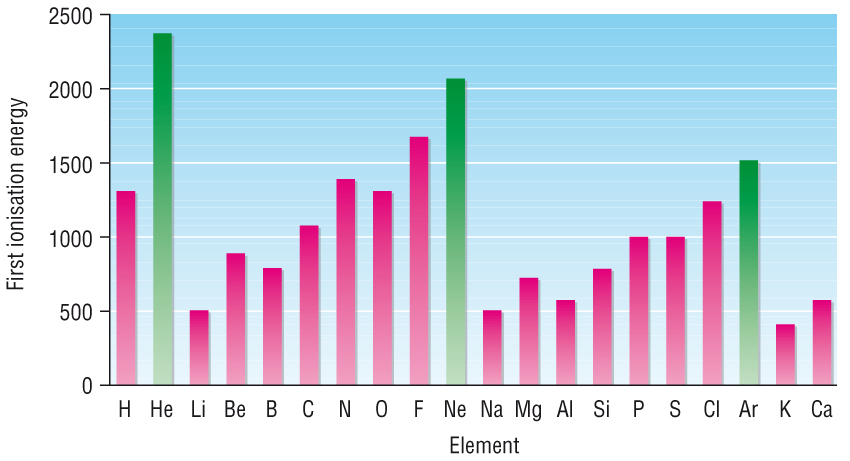
Recap – Factors affecting ionisation energies (from 1.2.1, P40)
1) The distance of the electron from the nucleus
2) Size of the positive nuclear charge
3) The ‘shielding’ effect by full inner shells
Explanation:
Start with atomic radii:
As you go across a period, the nuclear charge increases.
As the distance between nucleus and outer shell remains the same
And as shielding between nucleus and outer electron shell remains the same.
The increased nuclear charge pulls the electron shell closer to the nuclei.
This means there is a slightly greater attraction between the nucleus and the outer shell electrons.
Then include what effect that has on removing an electron:
This means that the first ionisation energy increases across a Period.
B) Trends down a Group:
1) Down a Group: Atomic radii increases:
 |
|
2) Down a Group: First ionisation energies decreases:
Explanation:
Start with atomic radii:
As you go down a group, the nuclear charge increases.
There are more electron shells which increases shielding between nucleus and outer electron shell.
As there are more electron shells, the distance between nucleus and outer shell increases.
This means that attraction between the nucleus and outer electrons decreases.
Then include what effect that has on removing an electron:
This means that the first ionisation energy decreases down a Group.
Questions 1 - 2 P85
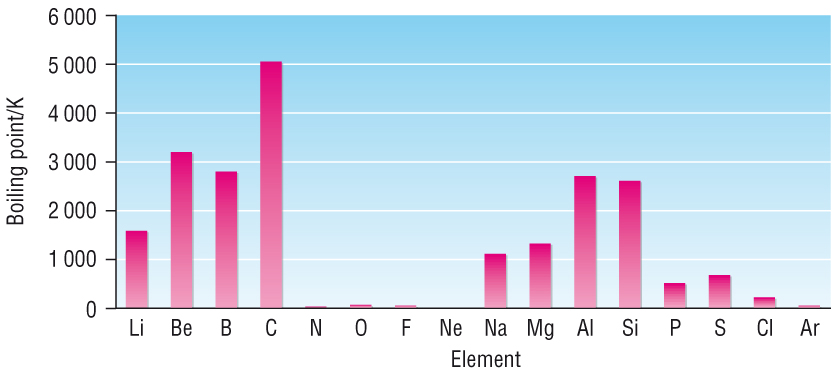
General trends:
Increase in Boiling point from Gp 1 - 4.
Sharp drop from Gp 4 - 5
Low Boiling points for Gp 5 - 0
Explanation:
As you move from left to right there is a general pattern:
Metals à Non - metals
And:
Solid à Gas
This means that there are different structures, forces of attraction and bonding.
All 3 of these must be considered when explaining boiling / melting points. As we move across a period.
Metallic à Giant Molecular à Molecular à Atomic
If you understand the structures, forces of attraction and bonding in all 4 of the above, then it becomes easier:
We shall take each one in turn:
Metallic bonding - Gp 1 - 3
The boiling points increase as you move along Group 1 - 3.
This is due to an increase in outer electrons available to be part of the mobile sea of electrons.
|
|
Giant covalent structures - Gp 4
These are carbon and silicon
This is due to the strong covalent bonds making up a giant structure.
For these to boil, the strong covalent bonds must be broken leaving single atoms.
|
Molecular / Atomic - Groups 5 - 0:
These are elements that are diatomic molecules (N2, O2) or single atoms (Ne, Ar).
The intermolecular forces between these elements are Van der Waals and therefore weak.
These Van der Waals are easily overcome separating the diatomic molecules or atoms from each other.
|
Summary:
|
|||||||||||||||||||||||||||||||||||||||||||||
Questions 1 - 2 P 87 / 1 - 3 P97 / 1 - 3 P98